>>> Vietnam consults experts on developing homegrown COVID-19 vaccines
>>> Vietnam to consider emergency licensing of first homegrown COVID-19 vaccine
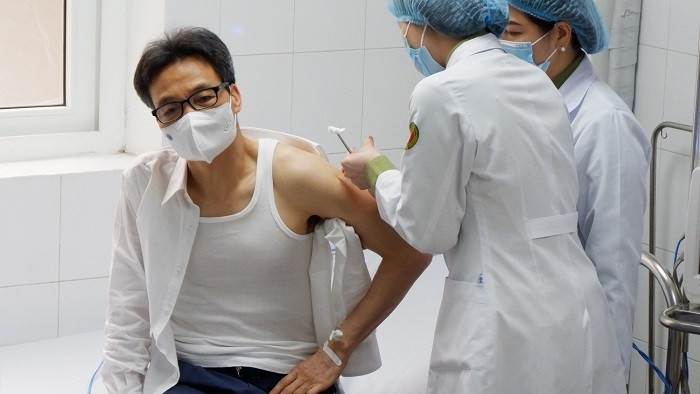
According to Professor, Dr. Tran Van Thuan, Deputy Minister of Health, official information on the latest trial results of the first made-in-Vietnam COVID-19 vaccine will be provided at the meeting.
Ho Nhan, the director of Nanogen Pharmaceutical Biotechnology Joint Stock Company and the vaccine’s developer, has sent an express dispatch to the Department of Science, Technology and Training under the Ministry of Health; the National Council on Ethics in Biomedical Research under Ministry of Health; the Ethics Council of Military Medical University; and the Ethical Committee of the Pasteur Institute in Ho Chi Minh City on the results of the trial results of Nano Covax with the protective effect based on the results of immunogenicity.
The research results were conducted by the Ho Chi Minh City Pasteur Institute, as part of the phase 2 study of the vaccine, in which scientists evaluated the virus-neutralising antibody titres by PRNT50 of people who had received the Nano Covax vaccine, compared with people with COVID-19 who had recovered from the disease.
Specifically, the research team took blood samples of 112 volunteers at 42 days after the injection of the first dose, and then performed a necrosis reduction neutralisation test (PRNT) with live SARS-CoV-2 virus on cell culture (PRNT 50) at the laboratory of the Pasteur Institute in Ho Chi Minh City.
The research team reported that the neutralising antibody titre of the Nano Covax vaccine is more than two times higher than that of the recovered group, equivalent to a 90% protective effect in preventing symptomatic COVID-19.
After three months of vaccination, the specific antibody level of Nano Covax injectors was still higher than that of the recovered group.
With the above results, Nanogen continued to ask the Ministry of Health and the Ethics Council for consideration to have an emergency licence for its vaccine.
The National Institute of Hygiene and Epidemiology is also evaluating its effectiveness against the Delta variant, with the initial results showing that the Nano Covax vaccine is capable of neutralising this deadly coronavirus variant.
Currently, the second dose of the Nano Covax vaccine is being injected for 12,000 volunteers participating in the phase 3b clinical trial, which is expected to be completed before August 15.
Earlier, at a meeting with Deputy Minister Tran Van Thuan on August 2, Nanogen company reported the results of analysis of blood samples on the 42nd day of 1,000 volunteers participating in phase 3a, which showed that 100% of them who injected the Nano Covax vaccine had Surrogate neutralising antibody at above 30%, with 99.2% of them having seroconversion of IgG antibodies against Protein S at four times higher.
The Ministry of Health suggested that Nanogen send a report on phase 2 and initial phase 3 research data before August 15, from which the ministry could report to the Ethics and Licensing Council for consideration of the authorisation for the vaccine in the state of emergency.